2020-03-19 10:20:40
Flavien Susanne, Benjamin Martin, Michel Aubry, Joerg Sedelmeier, Fabio Lima, Serbuelent Sevinc, Lorenzo Piccioni, Julien Haber, Berthold Schenkel, and Francesco Venturoni
Novartis Pharma AG, Chemical and Analytical Development, Fabrikstrasse 14, 4002, Basel, Switzerland
INTRODUCTION
Continuous manufacturing will increasingly be applied where there is both an interest in extending the palette of permissible reaction conditions and enabling reaction outcomes which are highly challenging with the incumbent batch technology.1 An opportunity in the emerging field of continuous manufacturing is the ability to match-make the ideal reactor to the chemistry in hand. In an established batch pilot plant, the opposite is more often the case, with the accompanying compromises in efficiency when quality-critical attributes and reactor capabilities do not align. Considering the smaller reactor volume and lower capital cost of continuous plants, a tailored approach can be implemented, whereby the parameters identified as critical by detailed process development can be addressed by a subsequent bespoke reactor design and construction phase. In addition, the detailed knowledge gained in the laboratory development phase can be used to appropriately allocate flow or batch techniques to the sequence of operations, from chemistry to work up, to purification.2 In this way a line is drawn between flow, semi-batch, and batch methods based on real process requirements and not on a desire to implement sequences in flow for their own sake.
Organometallic chemistry is a tremendous opportunity for continuous manufacturing3 and has been applied to demonstrated effect in the industrial arena. We have previously outlined a general methodology enabled by continuous flow processing toward substituted benzoxazoles starting from 3- halo-N-acyl anilines.5 The transformation proceeded via base- mediated deprotonation, ortho-lithiation, and intramolecular cyclization to provide unstable lithiated benzoxazoles which were subsequently trapped by a range of electrophiles, Scheme 1. Efficient heat removal, mixing, and avoiding holding-times were identified as critical parameters and were the underpinning for operating in tubular reactors with precooling loops at sub-ambient temperatures in continuous flow.
Within our pipeline of projects in chemical development, we identified a strong overlap with this methodology which could be used to increase the yield of a key benzoxazole building block. An active CXCR2 program in the chronic obstructive pulmonary disorder (COPD) disease area had undergone a synthesis evaluation phase, identifying optimal routes to the target squalamine receptor antagonist, Scheme 2.6 All selected routes required the benzoxazole 1 as starting material, and cost evaluation highlighted this compound as the key contributor to the final cost-of-goods of the drug substance. In addition, the supply chain for 1 was not robust, with an external contract manufacturing organization failing to meet the delivery request.
We, therefore, initiated the process research and development toward compound 1 and herein outline the transition from batch to flow, where appropriate, to achieve a more robust process with increased yield.
RESULTS AND DISCUSSION
Benzoxazole 1 is prepared from N-pivaloyl-protected 3,4- dichloro aniline 6 according to the chemical sequence summarized in Scheme 3.7 Under controlled conditions, the addition of 2 equiv of n-butyl lithium to aniline 6 leads to both deprotonation of the pivaloyl-amide (6a) and regioselective ortho-lithiation of the aromatic ring (6b). The ortho-lithiated intermediate is highly unstable and eliminates lithium chloride to create a transient aryne (6c) which is immediately quenched intramolecularly by the nucleophilic tautomer of the lithiated pivaloyl-amide to provide compound 7.8 Compound 7 is highly nucleophilic, and timely trapping with sulfur dioxide delivers the lithium sulfinate 8 which has sufficient stability to be isolated and characterized. The standard synthetic sequence to compound 1 continues however with a dual oxidation/ chlorination with sulfuryl chloride to provide the sulfonyl chloride 9. Compound 9 was shipped as a stable intermediate during early manufacturing campaigns, but since identification as a compound with genotoxicity potential is further transformed in the same reactor to the sulfonamide-substituted benzoxazole 1 by nucleophilic substitution with Weinreb’s amine, N,O-dimethylhydroxylamine. In batch mode, the internal temperature of the reactor for this one-pot sequence would be initially set to −30 °C, and a strong scale dependency was observed with accumulated side products needing purging by crystallization of 1. On a 30 g scale, an overall yield of 34% could be achieved in traditional batch mode.
First-Generation Continuous Process, Lab Scale. An initial lab-scale flow synthesis was devised in order to not only safely gain process familiarization with limited volumes but also determine if business benefits such as economy, yield, and safety could result from a straightforward continuous operation. As with our previous studies on model benzoxazoles, we selected tubular reactors and Teflon T-pieces in order to proceed with minimal complication and encounter the challenges as they presented themselves. A streamlined transition into continuous flow also enabled us to screen conditions “on-the-fly” which provided data much more rapidly than sequential batch reactions under cryogenic conditions ever could. As the chemistry proceeds stepwise through the sequence outlined in Scheme 3 the nature of the steps changes from fast highly exothermic reactions to compound 8 toward slower, less energetic functional group transformations to compound 1. As such we chose to break the sequence down into three parts for our lab-scale studies: (i) cryogenic n-butyl lithium chemistry; (ii) suflur dioxide quench followed by oxidation/chlorination to the sulfonyl chloride 9; and (iii) sulfonamide formation. We used our small-scale in-house automated flow reactor units for these studies, which we term the exploratory development units (ED-units).9 This allowed us to both program and track flow rates provided by dual-barrel continuous syringe pumps, as well as set safety limits for acceptable temperature and pressure fluctuations. Reactors were loops of perfluoroalkoxy (PFA) tubing, and mixers were PTFE T-pieces. These units have been used for screening and early phase clinical supplies for numerous years, and the degree of mixing in T-pieces has been well characterized.10 Although scale-up phenomenon will always apply, the ED-units permit a straightforward transition to our larger-scale units, termed process development units (PD-units), which have an identical control system but include pumps capable of higher throughput.
In order to study the first step with n-butyl lithium independently of the subsequent two steps, we selected acetic acid as an electrophile and targetted the stable proton-quench compound 10, Scheme 4. Precooling loops ensured that the set point of −20 °C could be attained in the streams prior to mixing in T-pieces. Following a screening to determine the optimum stoichiometry (2.4 equiv of Buli) and residence times (4.5 and 0.4 min respectively), the product 10 was obtained in 95% isolated yield and 99% selectivity.
With a reliable small-scale procedure for accessing lithiated intermediate 7 in hand, we switched the in-line electrophilic quench to a sulfur dioxide solution in THF, setting the temperature by experiment for this step to 0 °C. Semibatch quench of this combined stream was made into a solution of sulfuryl chloride in hexane at 5 °C to deliver sulfonyl chloride derivative 9 (general approach shown in Scheme 5). However, the partial insolubility of the sulfinate salt 8 formed after mixing with sulfur dioxide led to clogging of the PFA tubing, and so we resorted to ultrasound to disrupt the solid accumulation and keep the reaction medium flowing.11 This proved sufficient for a demo-run, successfully converting 30 g of starting material to compound 9. The first reactions with n-butyl lithium were considered a priori to be instantaneous, and so we were drawn to explore more efficient mixing in case this could streamline our process within the same ED-unit. A number of commercial micromixers were screened, but those with fine channels led to plugging. Staying within our remit of developing a lab-scale demonstration of the capabilities of flow processing, we found a pragmatic solution with a split-and-recombine reactor consisting simply of a T-piece to divide the stream immediately after n-butyl lithium addition and a T-piece to reunite the streams prior to an 11 mL residence volume coil (final conditions shown in Scheme 5). This effort brought the required residence time for the n-butyl lithium step down from 4.5 to 1.9 min allowing us to process a further 20 g of compound 6 over 4 h. This experiment confirmed that we had been, and most likely still were, highly mixing limited, but since scale-up of this laboratory setup was not our aim, we pushed forward to identify upcoming hurdles.
The final chemical step in the sequence to benzoxazole 1 was the conversion of the sulfonyl chloride 9 to the sulfonamide 1 by substitution with Weinreb’s amine. The crude sulfonyl chloride 9 coming from the flow synthesis contained significant amounts of both residual sulfur dioxide and sulfuryl chloride. In addition, and for reasons of convenience and safety,12 the Weinreb amine was purchased as a hydrochloric acid salt and therefore required deprotonation prior to reaction with 6. In order to limit the exothermic and avoid salt precipitation, we, therefore, opted for partial batch distillation of the reaction mixture to deplete the gaseous acidic components. The soluble reaction mixture could then be taken forward and combined with a basic aqueous solution of the Weinreb amine. Suitable bases were screened which were considered capable of deprotonating the amine (pKa 4.8) but hydrolyzing the sulfonyl chloride 9 to the sulfonic acid at only trace levels, finally settling upon dipotassium hydrogen phosphate.13 Screening of reaction residence time and temperature was conducted in the flow unit, with the use of a glass chicane mixer chip from Little Things Factory14 providing superior mixing of the organic and aqueous streams compared to an empty tube, Scheme 6. Operating at 80 °C the reaction required 25 min to reach full conversion, although this reaction time varied upward with increasing amounts of residual sulfur dioxide and sulfuryl chloride in the crude starting material stream.
Since the projected maximal commercial volume of the drug candidate was significantly less than 100 ton/annum, we decided that the workup and crystallization of compound 1 could be sufficiently addressed by batch operations. The product stream containing crude compound 1 was combined with toluene and aqueous citric acid. Upon mixing and separation of the phases the organic phase was extracted once more with sodium bicarbonate prior to a complete distillation solvent-switch to toluene down to a desired concentration. n- Heptane was added to the heated solution at 50 °C, and a cooling ramp brought about the crystallization. After washing the filter cake with toluene/heptane and subsequently drying, benzoxazole 1 was delivered as off-white crystals.
Having identified optimal conditions for a lab-scale synthesis of benzoxazole 1 in three continuous-parts, we proceeded to combine these operations into one continuous processing line. The resulting assembly is pictured in Figure 1 and contained three thermostated baths (one sonicated) and five pumps (four syringe-pumps and one Knaur pump). Since the sulfonamide formation is at the tail-end of the chemical sequence and has, in any case, the slowest kinetics of the transformations, a 200 mL wide-bore (1/4″) PFA tube was used to enable complete
conversion, with insertion of static mixer elements to ensure
sufficient mixing efficiency, plug flow, and heat-transfer.
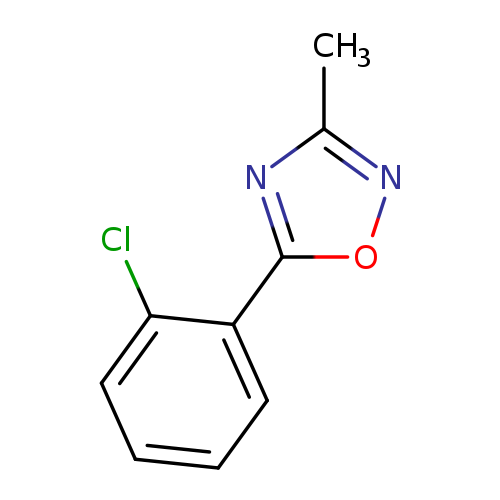
5-(2-Chlorophenyl)-3-methyl-1,2,4-oxadiazoleCatalog No.:AA00HC9O CAS No.:1120271-16-0 MDL No.:MFCD13961846 MF:C9H7ClN2O MW:194.6177 |
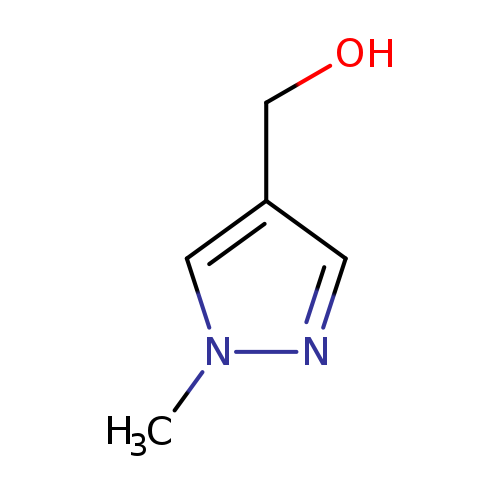
(1-Methylpyrazol-4-yl)methanolCatalog No.:AA008U5T CAS No.:112029-98-8 MDL No.:MFCD01822311 MF:C5H8N2O MW:112.1298 |
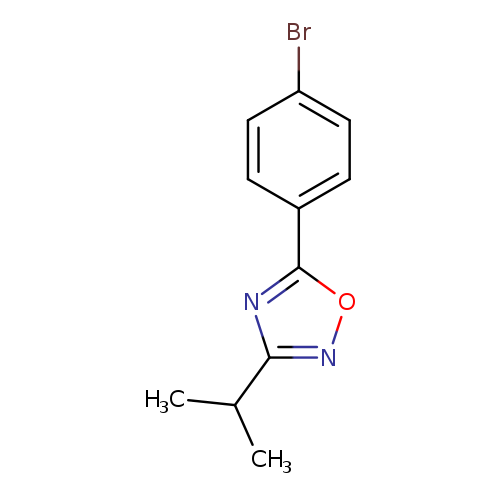
5-(4-bromophenyl)-3-(propan-2-yl)-1,2,4-oxadiazoleCatalog No.:AA01AIUL CAS No.:1120294-70-3 MDL No.:MFCD13881739 MF:C11H11BrN2O MW:267.1218 |
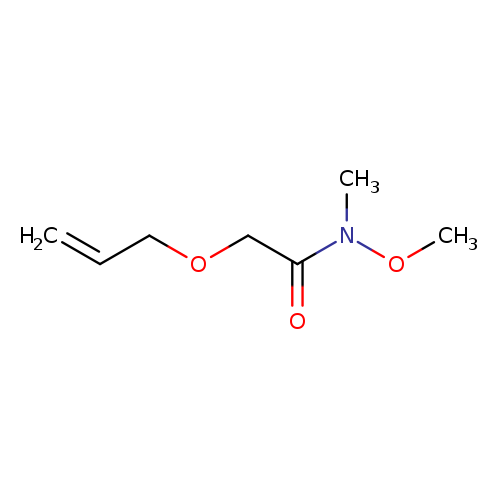
2-(Allyloxy)-N-methoxy-N-methylacetamideCatalog No.:AA00HC9P CAS No.:1120309-29-6 MDL No.:MFCD29059382 MF:C7H13NO3 MW:159.1830 |
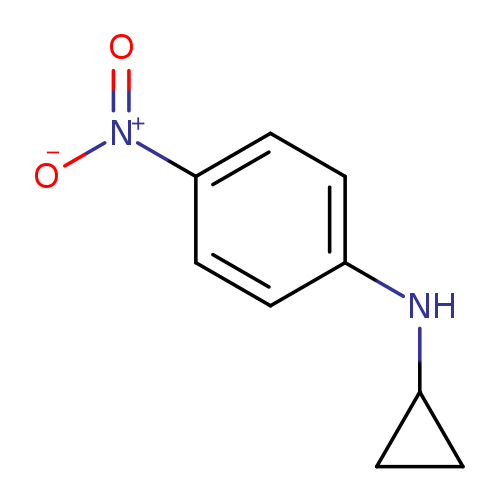
N-cyclopropyl-4-nitroanilineCatalog No.:AA01A63K CAS No.:112033-47-3 MDL No.:MFCD11122421 MF:C9H10N2O2 MW:178.1879 |
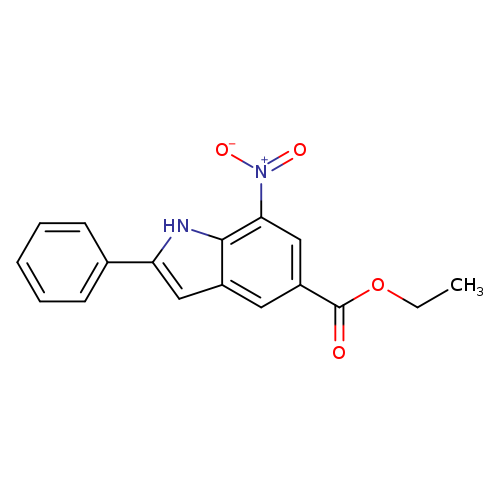
ethyl 7-nitro-2-phenyl-1H-indole-5-carboxylateCatalog No.:AA019EZV CAS No.:1120334-30-6 MDL No.:MFCD31652813 MF:C17H14N2O4 MW:310.3041 |
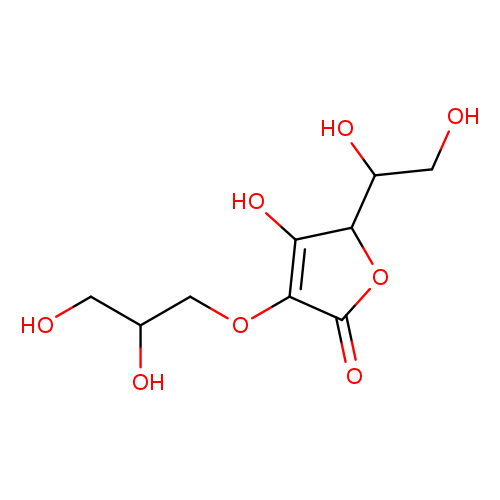
Glyceryl AscorbateCatalog No.:AA00943A CAS No.:1120360-13-5 MDL No.:MFCD28386108 MF:C9H14O8 MW:250.2027 |
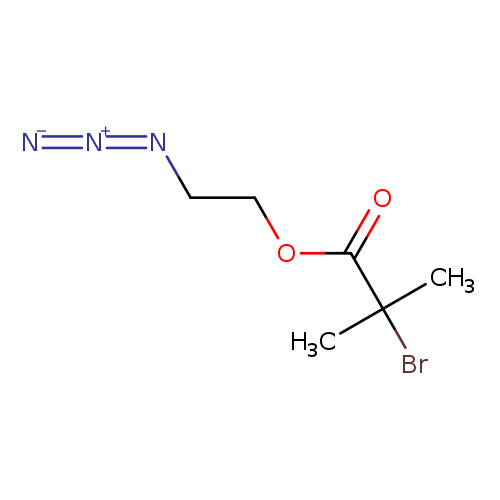
2'-Azidoethyl 2-bromoisobutyrateCatalog No.:AA0096C7 CAS No.:1120364-53-5 MDL No.:MFCD28144595 MF:C6H10BrN3O2 MW:236.0665 |
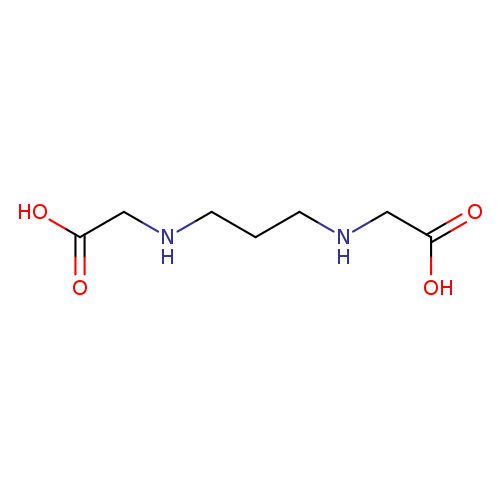
1,3-Diaminopropane-n,n'-diacetic acidCatalog No.:AA003DFY CAS No.:112041-05-1 MDL No.:MFCD00142534 MF:C7H14N2O4 MW:190.1971 |
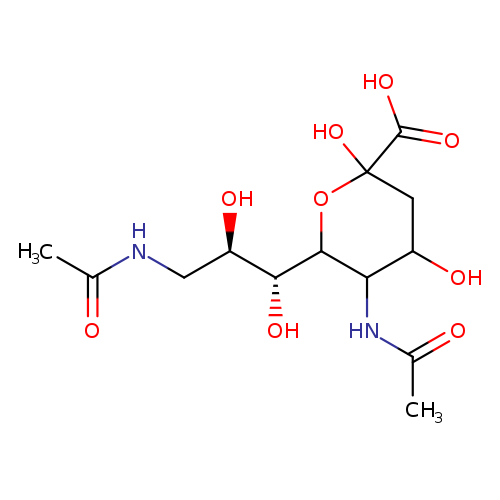
N-Acetyl-9-(acetylaMino)-9-deoxyneuraMinic AcidCatalog No.:AA008WI6 CAS No.:112054-78-1 MDL No.: MF:C13H22N2O9 MW:350.3218 |
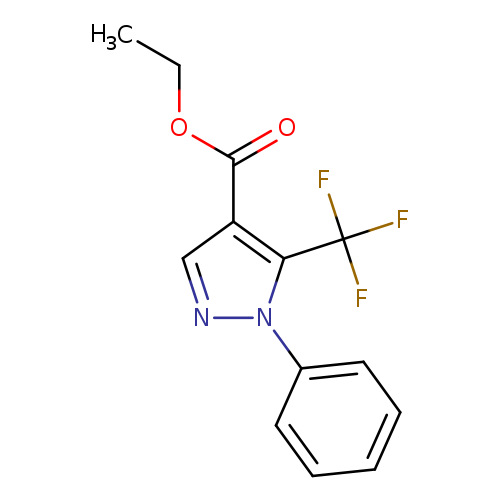
Ethyl 1-phenyl-5-(trifluoromethyl)pyrazole-4-carboxylateCatalog No.:AA008R7D CAS No.:112055-34-2 MDL No.:MFCD00068138 MF:C13H11F3N2O2 MW:284.2338 |
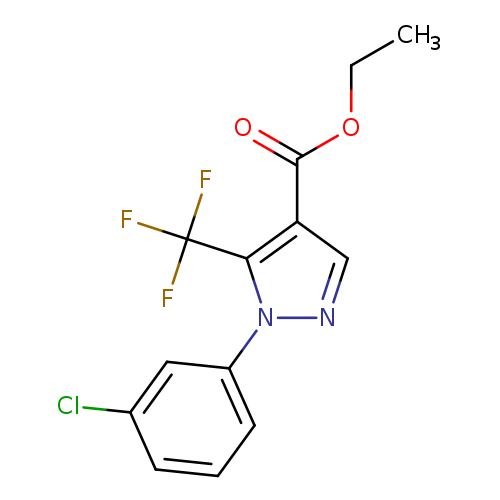
Ethyl 1-(3-chlorophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylateCatalog No.:AA007CR1 CAS No.:112055-35-3 MDL No.:MFCD03934803 MF:C13H10ClF3N2O2 MW:318.6789 |
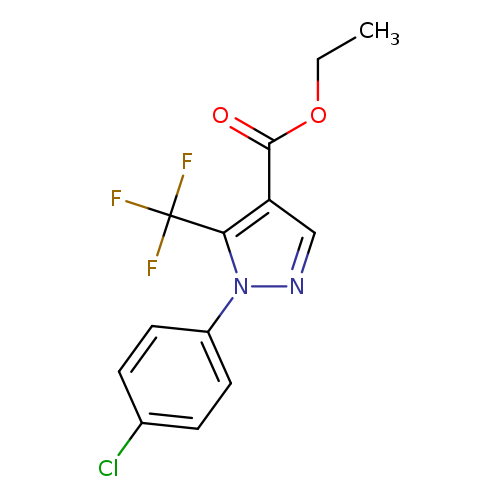
Ethyl 1-(4-chlorophenyl)-5-(trifluoromethyl)-1h-pyrazole-4-carboxylateCatalog No.:AA007UO3 CAS No.:112055-36-4 MDL No.:MFCD00052074 MF:C13H10ClF3N2O2 MW:318.6789 |
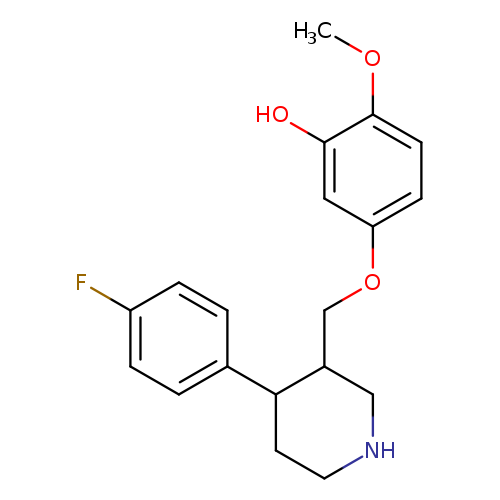
4-(4-Fluorophenyl)-3-(4-methoxy-3-hydroxyphenoxymethyl)piperidineCatalog No.:AA008X44 CAS No.:112058-89-6 MDL No.:MFCD11973661 MF:C19H22FNO3 MW:331.3813 |
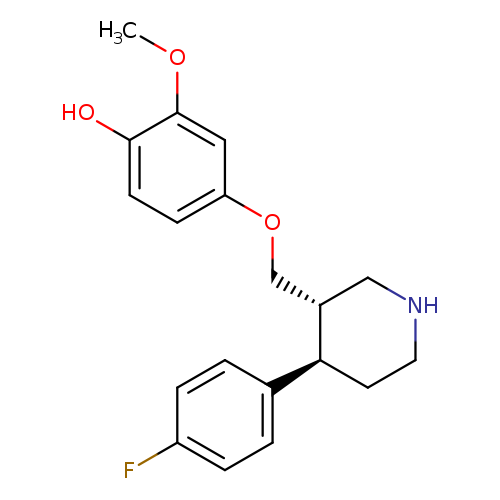
Phenol,4-[[(3S,4R)-4-(4-fluorophenyl)-3-piperidinyl]methoxy]-2-methoxy-Catalog No.:AA007UNX CAS No.:112058-90-9 MDL No.:MFCD11973662 MF:C19H22FNO3 MW:331.3813 |
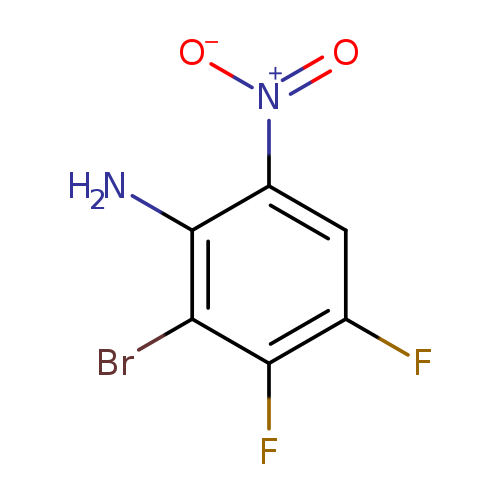
2-Bromo-3,4-difluoro-6-nitro-phenylamineCatalog No.:AA0096Y2 CAS No.:112062-62-1 MDL No.:MFCD20441752 MF:C6H3BrF2N2O2 MW:253.0010 |
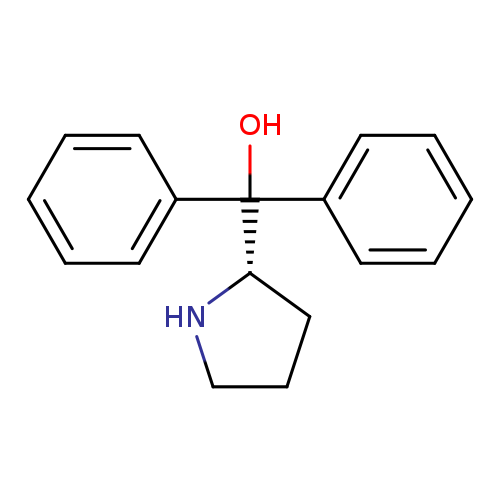
(S)-1,1-DiphenylprolinolCatalog No.:AA003CB8 CAS No.:112068-01-6 MDL No.:MFCD00075506 MF:C17H19NO MW:253.3389 |
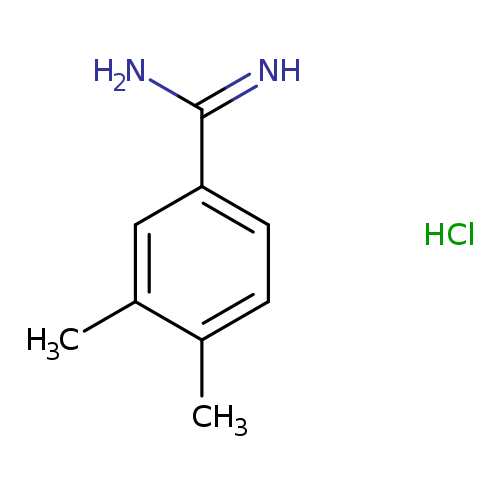
3,4-Dimethyl-benzamidine hydrochlorideCatalog No.:AA008T3X CAS No.:112072-09-0 MDL No.:MFCD08059236 MF:C9H13ClN2 MW:184.6659 |
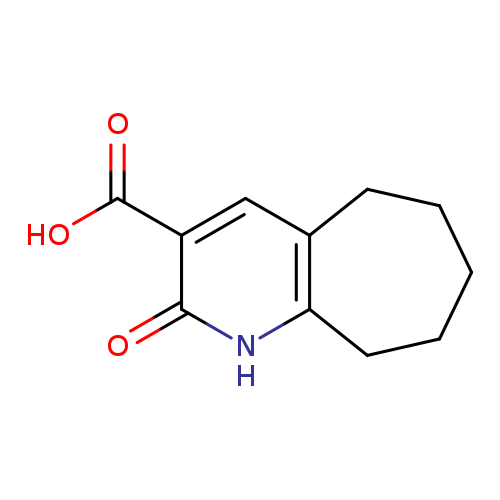
2-Oxo-1h,2h,5h,6h,7h,8h,9h-cyclohepta[b]pyridine-3-carboxylic acidCatalog No.:AA008S9D CAS No.:112072-32-9 MDL No.:MFCD07186450 MF:C11H13NO3 MW:207.2258 |
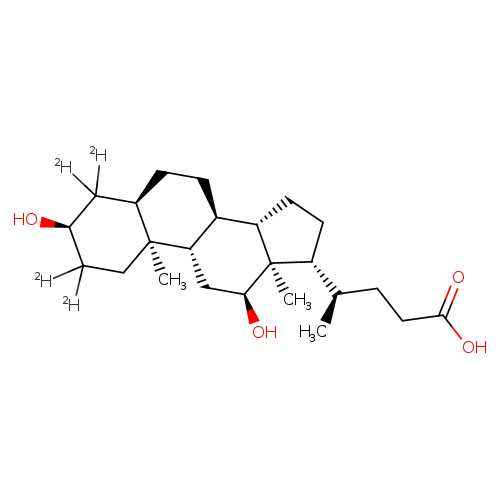
DEOXYCHOLIC-2,2,4,4-D4 ACIDCatalog No.:AA008SYD CAS No.:112076-61-6 MDL No.:MFCD00209707 MF:C24H36D4O4 MW:396.5966 |
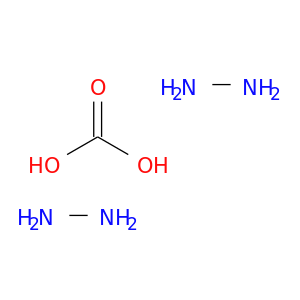
HYDRAZINE CARBONATECatalog No.:AA003QVO CAS No.:112077-84-6 MDL No.:MFCD00185569 MF: MW: |
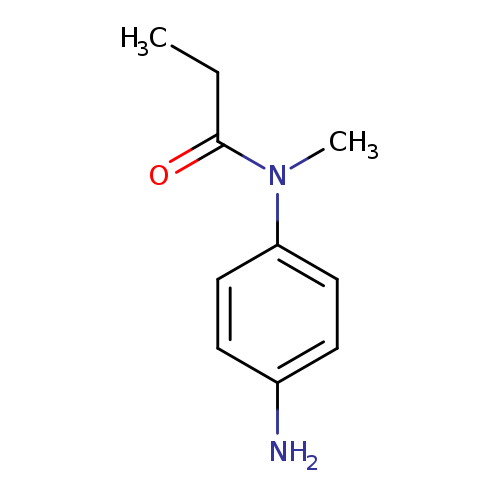
N-(4-aminophenyl)-n-methylpropanamideCatalog No.:AA009U3F CAS No.:112077-95-9 MDL No.:MFCD11184901 MF:C10H14N2O MW:178.2310 |

1,6,7,12-Tetrakis(4-tert-butylphenoxy)-N,N'-bis(2,6-diisopropylphenyl)-3,4,9,10-perylenetetracarboxylic DiiMideCatalog No.:AA008WKJ CAS No.:112078-08-7 MDL No.:MFCD15145478 MF:C88H90N2O8 MW:1303.6648 |
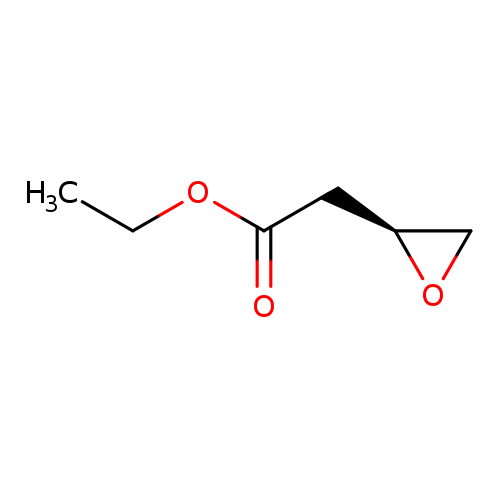
Ethyl (S)-2-oxiranylacetateCatalog No.:AA003PX9 CAS No.:112083-63-3 MDL No.:MFCD00672889 MF:C6H10O3 MW:130.1418 |
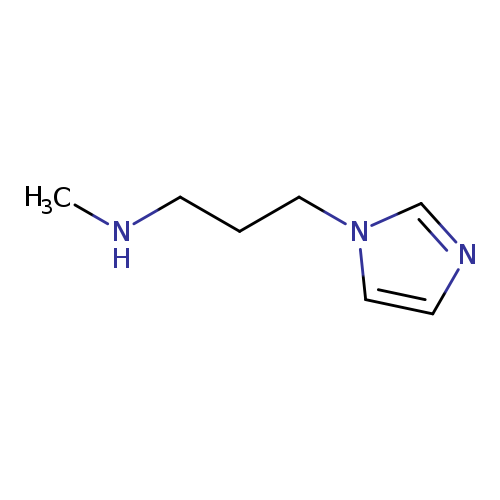
[3-(1H-imidazol-1-yl)propyl](methyl)amineCatalog No.:AA01A113 CAS No.:112086-54-1 MDL No.:MFCD09031545 MF:C7H13N3 MW:139.1982 |
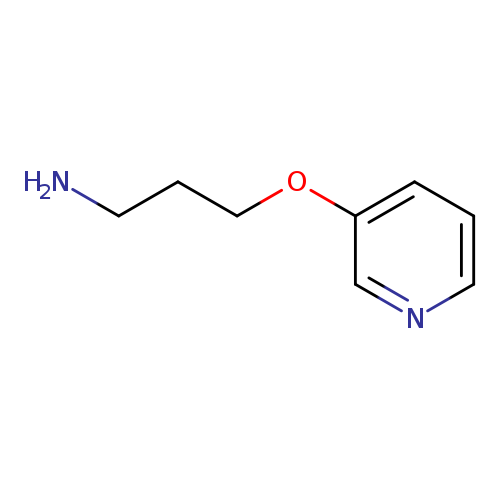
3-(Pyridin-3-yloxy)propan-1-amineCatalog No.:AA0083IT CAS No.:112086-55-2 MDL No.:MFCD08059775 MF:C8H12N2O MW:152.1937 |
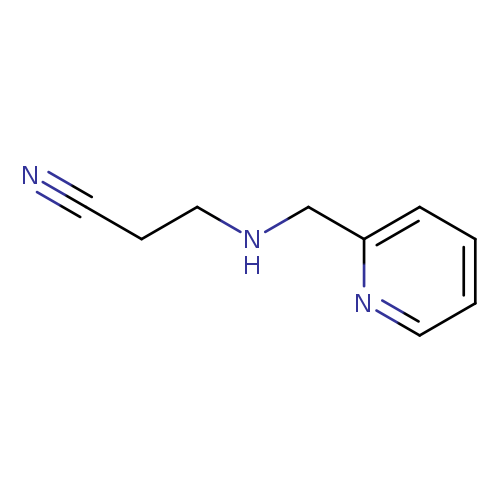
3-[(Pyridin-2-ylmethyl)amino]propanenitrileCatalog No.:AA01AALR CAS No.:112086-58-5 MDL No.:MFCD11166782 MF:C9H11N3 MW:161.2037 |
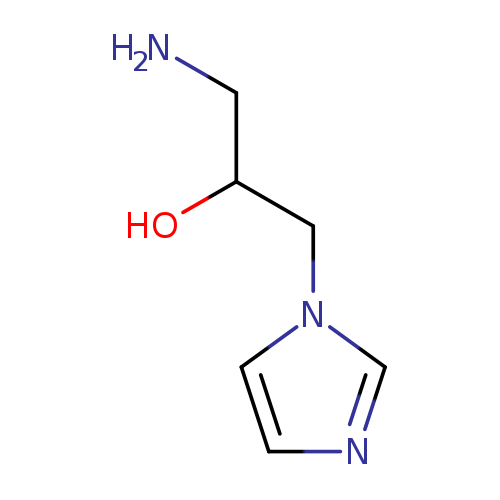
1-Amino-3-(1h-imidazol-1-yl)propan-2-olCatalog No.:AA01DUWP CAS No.:112086-60-9 MDL No.:MFCD09036065 MF:C6H11N3O MW:141.1710 |
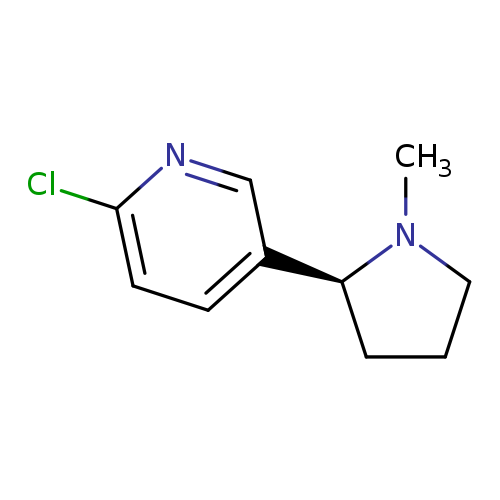
(S)-2-Chloro-5-(1-methylpyrrolidin-2-yl)pyridineCatalog No.:AA008VPL CAS No.:112091-17-5 MDL No.:MFCD22577282 MF:C10H13ClN2 MW:196.6766 |
Ethyl chloro[2-(4-chloro-2-nitrophenyl)hydrazono]acetateCatalog No.:AA008VB7 CAS No.:112091-27-7 MDL No.:MFCD06200942 MF:C10H9Cl2N3O4 MW:306.1022 |
1-{3-[2-(3-acetylphenoxy)ethoxy]phenyl}ethan-1-oneCatalog No.:AA019XG8 CAS No.:112092-59-8 MDL No.:MFCD13196178 MF:C18H18O4 MW:298.3331 |
(Z)-4-Hydroxy-N-desmethyl Tamoxifen (mixture of isomers)Catalog No.:AA007UN2 CAS No.:112093-28-4 MDL No.:MFCD09840374 MF:C25H27NO2 MW:373.4874 |
3-Methyl-1,2-oxathiolane 2,2-dioxideCatalog No.:AA00HC9T CAS No.:1121-03-5 MDL No.:MFCD01941639 MF:C4H8O3S MW:136.1695 |
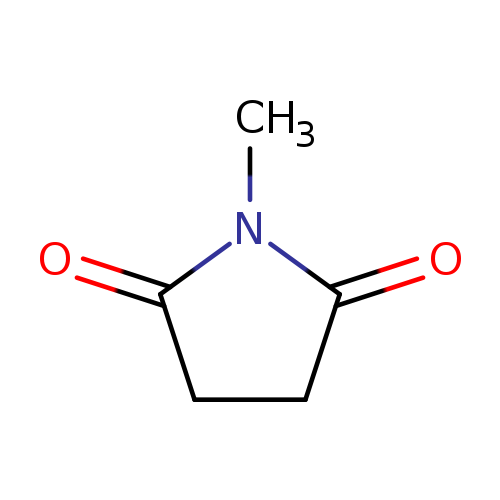
N-MethylsuccinimideCatalog No.:AA0035BV CAS No.:1121-07-9 MDL No.:MFCD00005517 MF:C5H7NO2 MW:113.1146 |
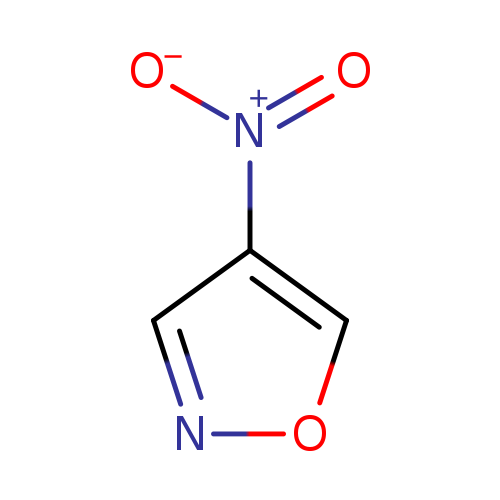
4-NitroisoxazoleCatalog No.:AA007UE0 CAS No.:1121-13-7 MDL No.:MFCD06638027 MF:C3H2N2O3 MW:114.0596 |
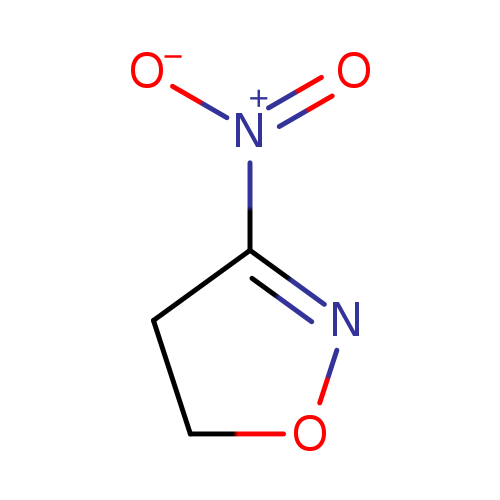
3-Nitro-4,5-dihydroisoxazoleCatalog No.:AA0083C9 CAS No.:1121-14-8 MDL No.:MFCD11878031 MF:C3H4N2O3 MW:116.0755 |
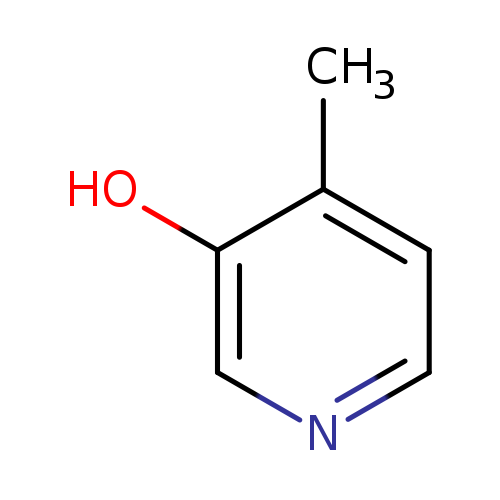
3-Hydroxy-4-methylpyridineCatalog No.:AA003JH5 CAS No.:1121-19-3 MDL No.:MFCD04114227 MF:C6H7NO MW:109.1259 |
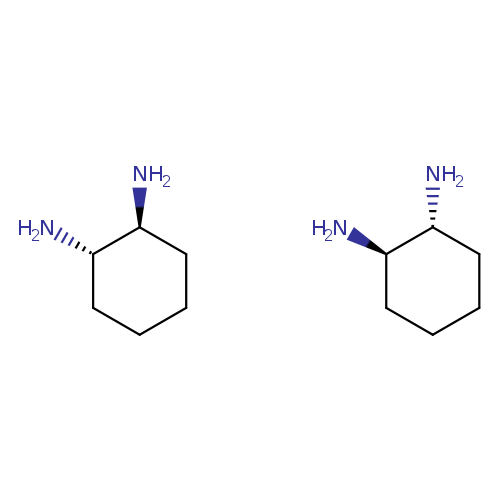
(+/-)-Trans-1,2-diaminocyclohexaneCatalog No.:AA00HC9U CAS No.:1121-22-8 MDL No.:MFCD00063747 MF:C12H28N4 MW:228.3775 |
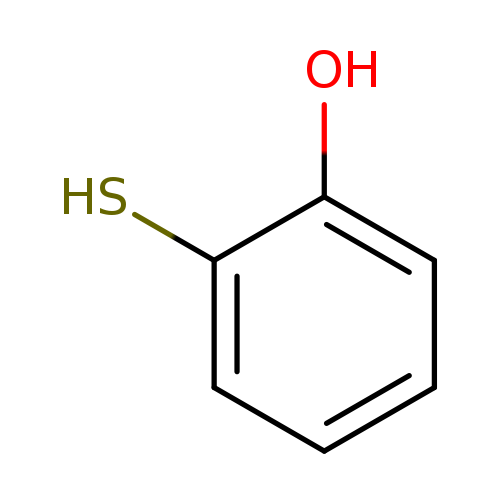
2-MercaptophenolCatalog No.:AA003HFM CAS No.:1121-24-0 MDL No.:MFCD00040447 MF:C6H6OS MW:126.1762 |
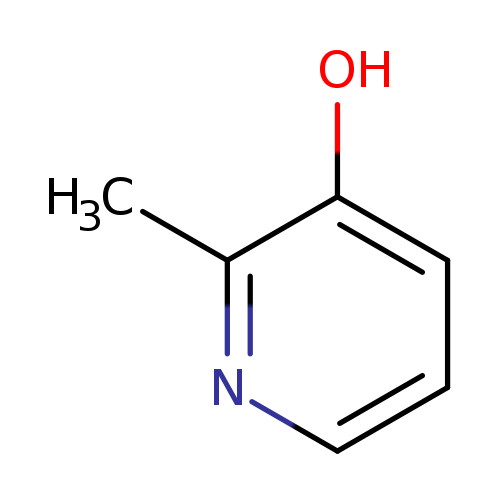
3-Hydroxy-2-methylpyridineCatalog No.:AA0033N4 CAS No.:1121-25-1 MDL No.:MFCD00082538 MF:C6H7NO MW:109.1259 |
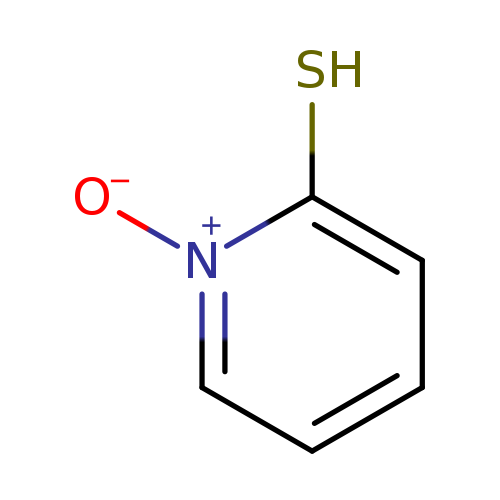
2-Mercaptopyridine-N-oxideCatalog No.:AA003HUE CAS No.:1121-31-9 MDL No.:MFCD00006196 MF:C5H5NOS MW:127.1643 |
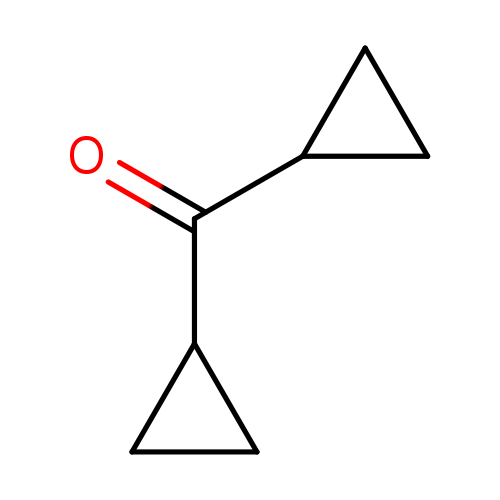
Dicyclopropyl KetoneCatalog No.:AA003PBN CAS No.:1121-37-5 MDL No.:MFCD00001276 MF:C7H10O MW:110.1537 |
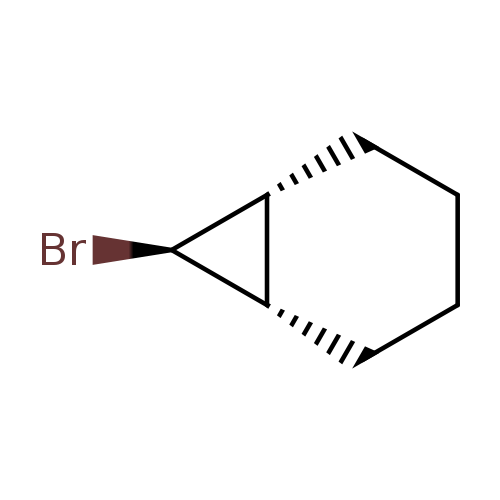
(1R,6S,7R)-7-bromobicyclo[4.1.0]heptaneCatalog No.:AA01DUWQ CAS No.:1121-41-1 MDL No.:MFCD31629001 MF:C7H11Br MW:175.0662 |
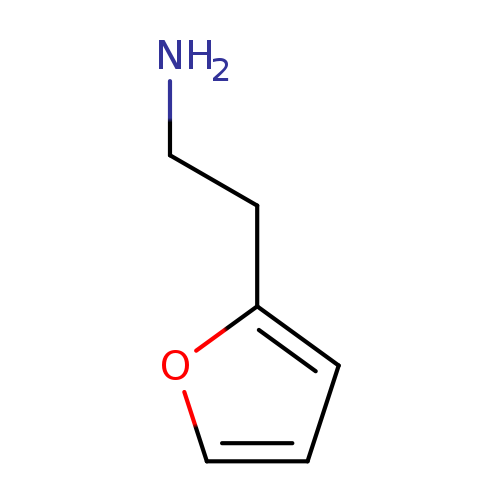
2-Furan-2-yl-ethylamineCatalog No.:AA003H9S CAS No.:1121-46-6 MDL No.:MFCD04113973 MF:C6H9NO MW:111.1418 |
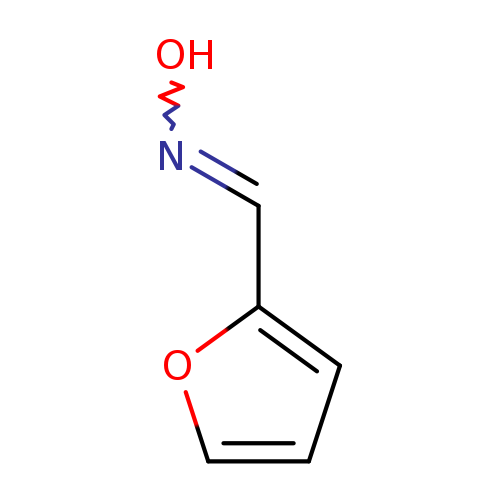
2-Furaldehyde oximeCatalog No.:AA007C2Q CAS No.:1121-47-7 MDL No.:MFCD00014114 MF:C5H5NO2 MW:111.0987 |
3-VinylpyridineCatalog No.:AA0033PL CAS No.:1121-55-7 MDL No.:MFCD02178019 MF:C7H7N MW:105.1372 |
4-MethylaminopyridineCatalog No.:AA007C2J CAS No.:1121-58-0 MDL No.:MFCD00152625 MF:C6H8N2 MW:108.1411 |
2-PyridinecarboxaldehydeCatalog No.:AA0033CY CAS No.:1121-60-4 MDL No.:MFCD00006290 MF:C6H5NO MW:107.1100 |
4-Chloromethyl-[1,3]dioxaneCatalog No.:AA00838G CAS No.:1121-62-6 MDL No.:MFCD00622396 MF:C5H9ClO2 MW:136.5768 |
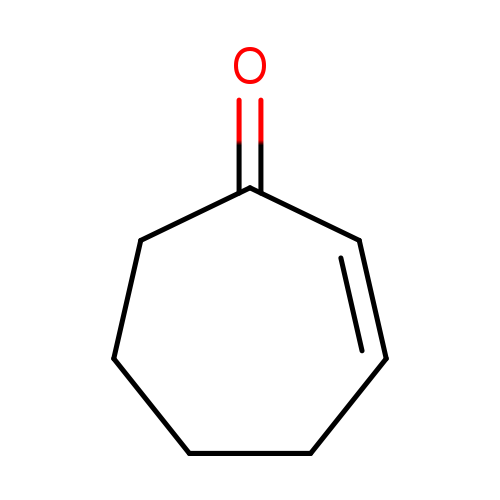
Cyclohept-2-en-1-oneCatalog No.:AA008R80 CAS No.:1121-66-0 MDL No.:MFCD00004157 MF:C7H10O MW:110.1537 |
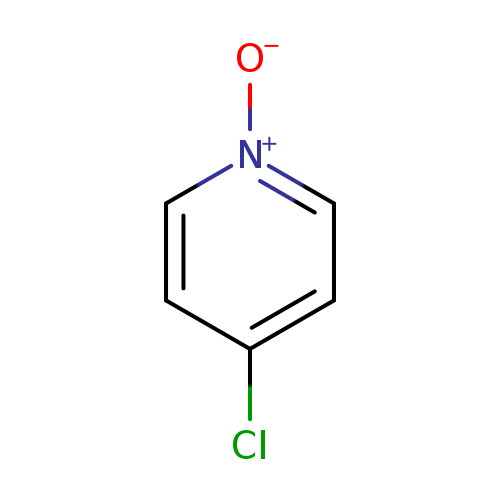
4-Chloropyridine n-oxideCatalog No.:AA003L5K CAS No.:1121-76-2 MDL No.:MFCD00047425 MF:C5H4ClNO MW:129.5444 |
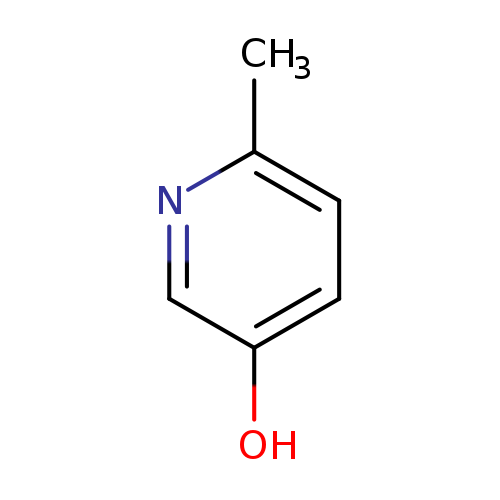
6-Methylpyridin-3-olCatalog No.:AA003JHB CAS No.:1121-78-4 MDL No.:MFCD29044409 MF:C6H7NO MW:109.1259 |
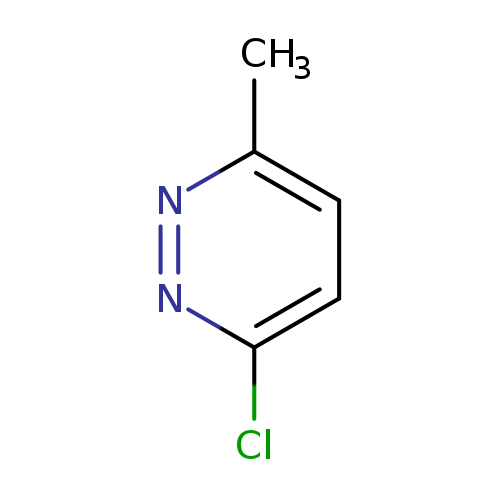
3-Chloro-6-methylpyridazineCatalog No.:AA003J7B CAS No.:1121-79-5 MDL No.:MFCD00052905 MF:C5H5ClN2 MW:128.5596 |
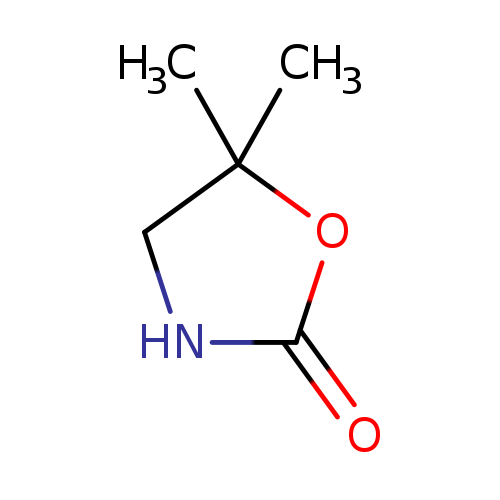
5,5-Dimethyloxazolidin-2-oneCatalog No.:AA00HC9V CAS No.:1121-83-1 MDL No.:MFCD11502070 MF:C5H9NO2 MW:115.1305 |
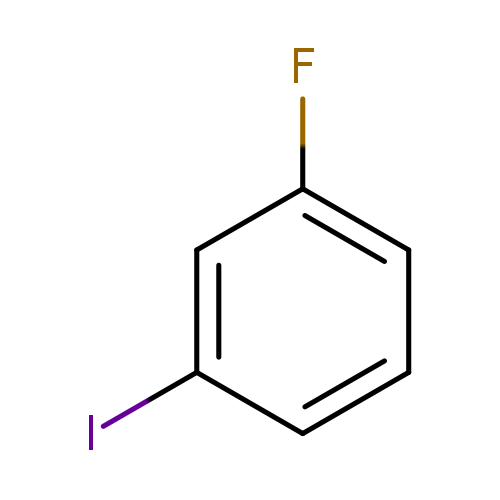
1-Fluoro-3-iodobenzeneCatalog No.:AA0032OO CAS No.:1121-86-4 MDL No.:MFCD00001044 MF:C6H4FI MW:221.9988 |
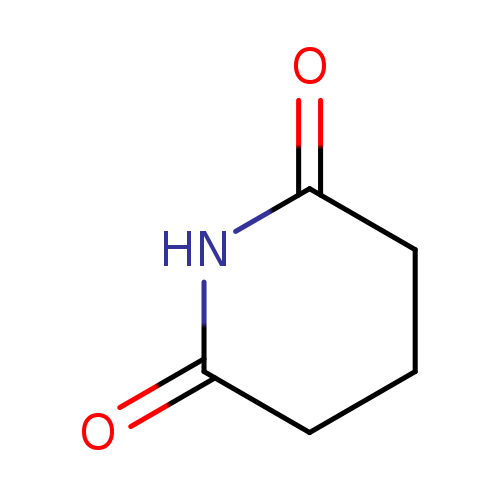
GlutarimideCatalog No.:AA003QP4 CAS No.:1121-89-7 MDL No.:MFCD00006670 MF:C5H7NO2 MW:113.1146 |
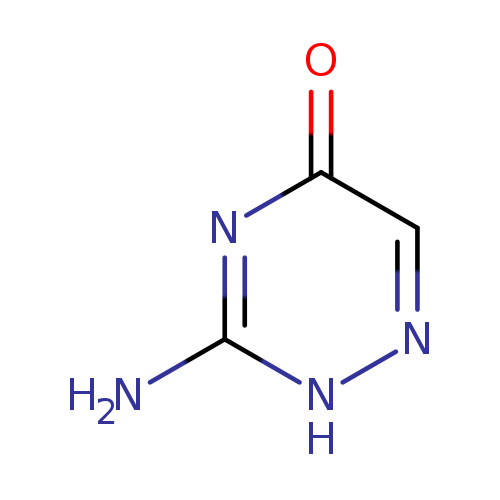
2,4-Dihydroxy-5-iodobenzoic acidCatalog No.:AA01A3QQ CAS No.:1121-90-0 MDL No.:MFCD19236953 MF:C3H4N4O MW:112.0901 |
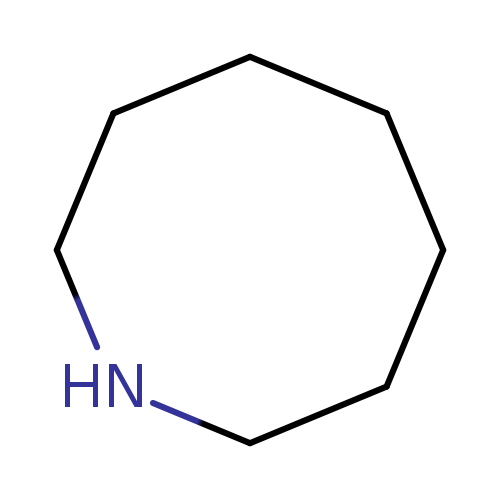
HeptamethyleneimineCatalog No.:AA007BY6 CAS No.:1121-92-2 MDL No.:MFCD00003270 MF:C7H15N MW:113.2007 |
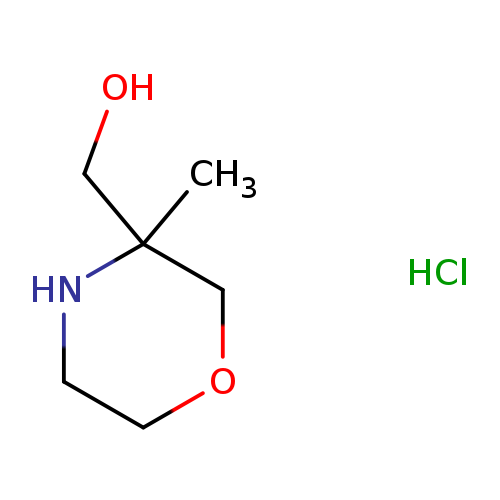
(3-Methylmorpholin-3-yl)methanol hydrochlorideCatalog No.:AA01A5JB CAS No.:1121-98-8 MDL No.:MFCD24842964 MF:C6H14ClNO2 MW:167.6339 |
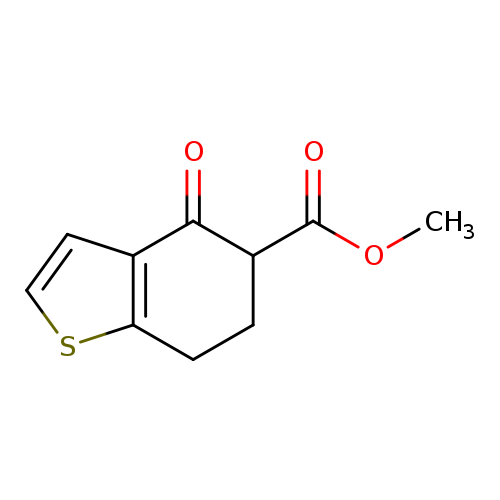
Methyl 4-oxo-4,5,6,7-tetrahydrobenzo[b]thiophene-5-carboxylateCatalog No.:AA019EJS CAS No.:112101-60-7 MDL No.:MFCD00137807 MF:C10H10O3S MW:210.2496 |
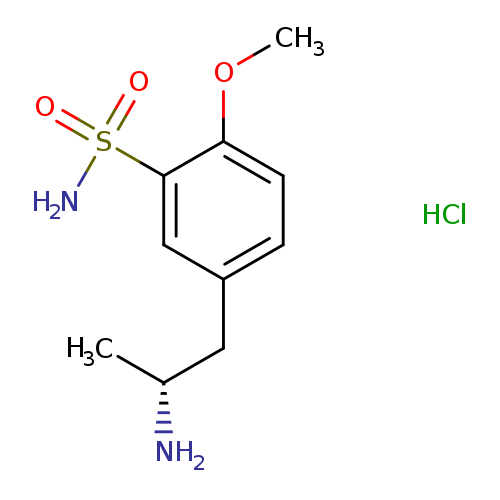
(R)-(+)-5-(2-Aminopropyl)-2-methoxybenzene sulfonamide hydrochlorideCatalog No.:AA007UMV CAS No.:112101-75-4 MDL No.:MFCD08460105 MF:C10H17ClN2O3S MW:280.7716 |
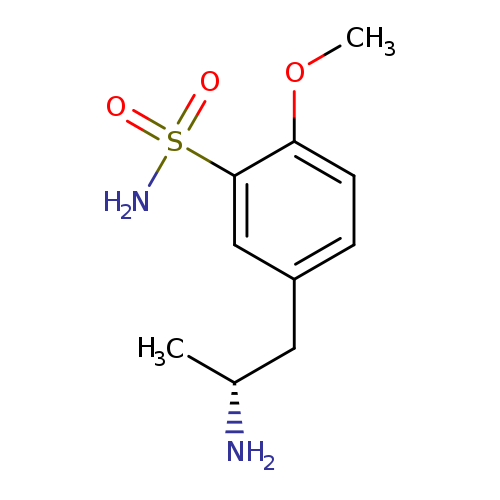
R-(-)-5-(2-Amino-propyl)-2-methoxy-benzenesulfonamideCatalog No.:AA0083CS CAS No.:112101-81-2 MDL No.:MFCD07782137 MF:C10H16N2O3S MW:244.3106 |
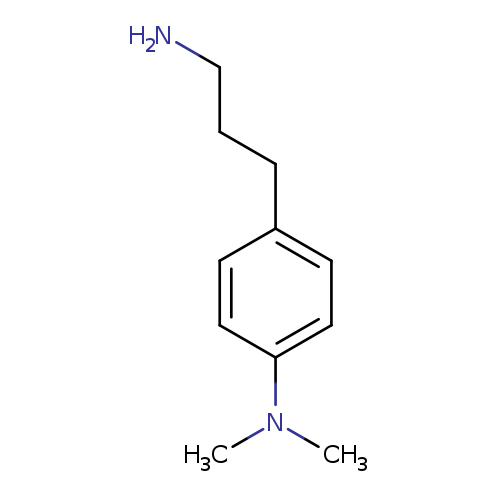
4-(3-aminopropyl)-N,N-dimethylanilineCatalog No.:AA019M8A CAS No.:112103-97-6 MDL No.:MFCD09886638 MF:C11H18N2 MW:178.2740 |
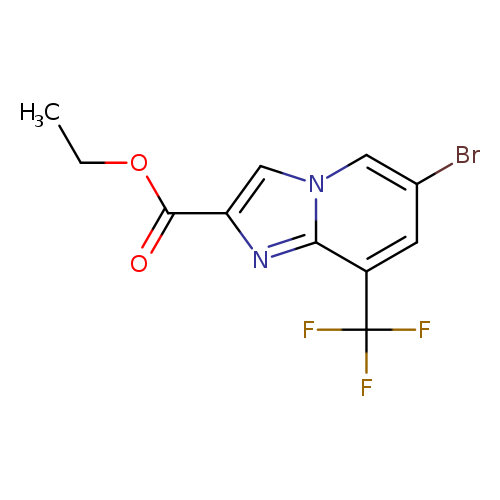
Ethyl 6-bromo-8-(trifluoromethyl)imidazo[1,2-a]pyridine-2-carboxylateCatalog No.:AA0093L7 CAS No.:1121051-30-6 MDL No.:MFCD19704051 MF:C11H8BrF3N2O2 MW:337.0926 |
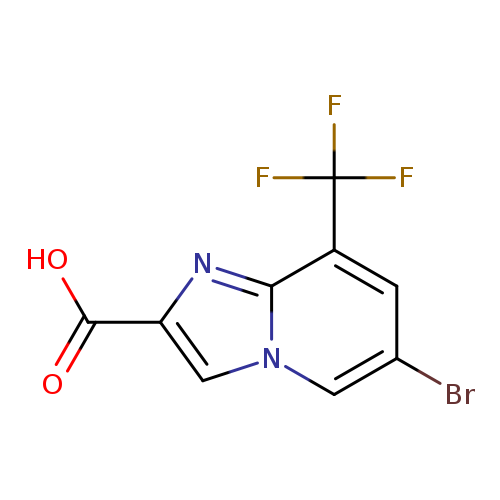
6-Bromo-8-(trifluoromethyl)imidazo[1,2-a]pyridine-2-carboxylic acidCatalog No.:AA0093L6 CAS No.:1121051-31-7 MDL No.:MFCD19687630 MF:C9H4BrF3N2O2 MW:309.0395 |
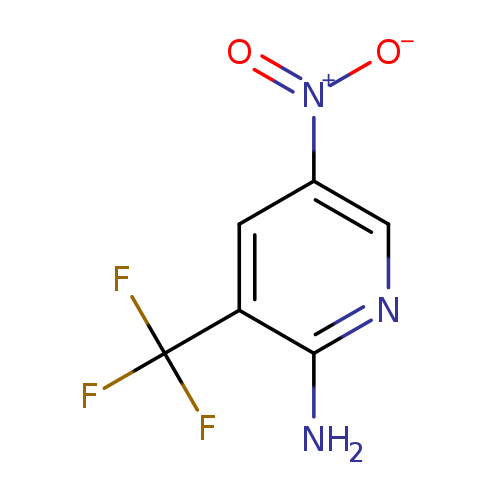
5-Nitro-3-(trifluoromethyl)pyridin-2-amineCatalog No.:AA007CBT CAS No.:1121056-94-7 MDL No.:MFCD18382741 MF:C6H4F3N3O2 MW:207.1101 |
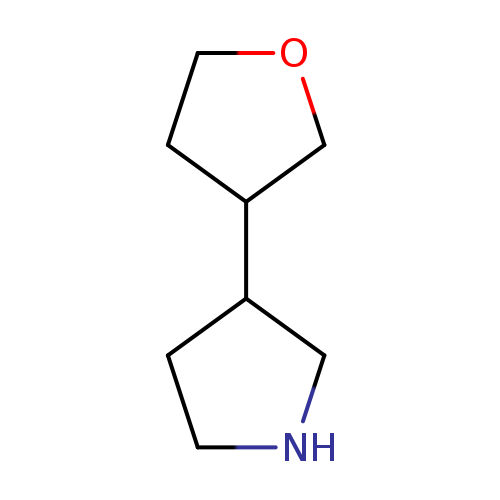
3-(oxolan-3-yl)pyrrolidineCatalog No.:AA01A1TP CAS No.:1121057-47-3 MDL No.:MFCD16707136 MF:C8H15NO MW:141.2108 |
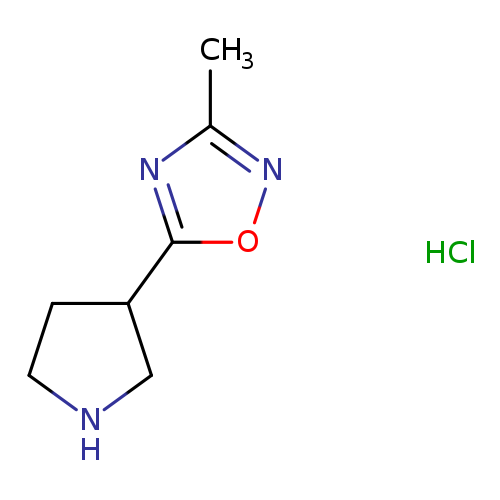
3-Methyl-5-(3-pyrrolidinyl)-1,2,4-oxadiazole hydrochlorideCatalog No.:AA00907Z CAS No.:1121057-52-0 MDL No.:MFCD11870728 MF:C7H12ClN3O MW:189.6427 |
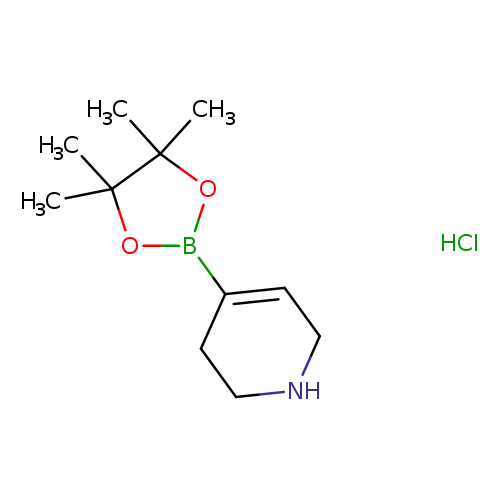
1,2,3,6-Tetrahydropyridine-4-boronic acid, pinacol ester, HClCatalog No.:AA003901 CAS No.:1121057-75-7 MDL No.:MFCD11506076 MF:C11H21BClNO2 MW:245.5539 |
tert-Butyl 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2h)-carboxylateCatalog No.:AA0095WY CAS No.:1121057-77-9 MDL No.:MFCD18383341 MF:C16H28BNO4 MW:309.2088 |
1H-Indole-7-carboxylic acid,2,3-dihydro-,methyl esterCatalog No.:AA0083CN CAS No.:112106-91-9 MDL No.:MFCD09954772 MF:C10H11NO2 MW:177.1998 |
1-(2-Chloro-4-fluorophenyl)ethanolCatalog No.:AA007CBO CAS No.:112108-68-6 MDL No.:MFCD04973765 MF:C8H8ClFO MW:174.5999 |
2-Chloro-4-fluoro-5-nitrotolueneCatalog No.:AA0032O1 CAS No.:112108-73-3 MDL No.:MFCD11110549 MF:C7H5ClFNO2 MW:189.5715 |
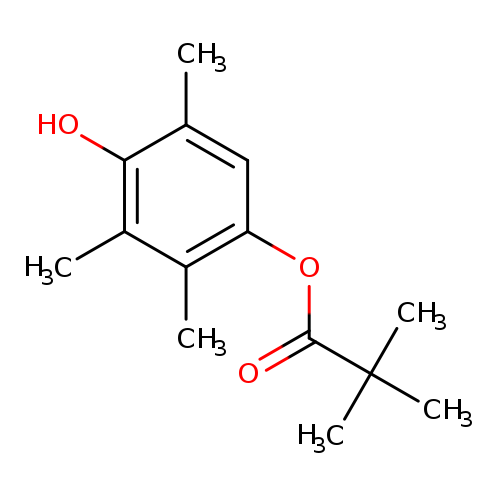
1-PIVALOYL-2,3,5-TRIMETHYLHYDROQUINONECatalog No.:AA008W08 CAS No.:112109-69-0 MDL No.:MFCD09841194 MF:C14H20O3 MW:236.3068 |
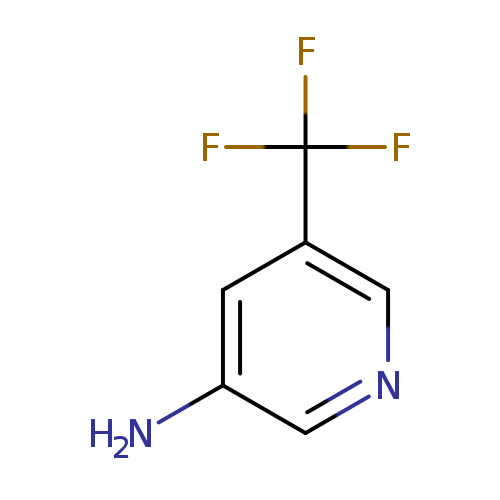
5-(trifluoromethyl)pyridin-3-amineCatalog No.:AA007CBM CAS No.:112110-07-3 MDL No.:MFCD00128900 MF:C6H5F3N2 MW:162.1125 |
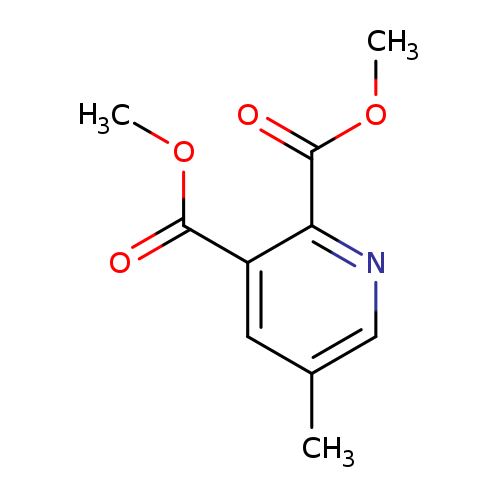
Dimethyl 5-methylpyridine-2,3-dicarboxylateCatalog No.:AA00HC9W CAS No.:112110-16-4 MDL No.:MFCD05662424 MF:C10H11NO4 MW:209.1986 |
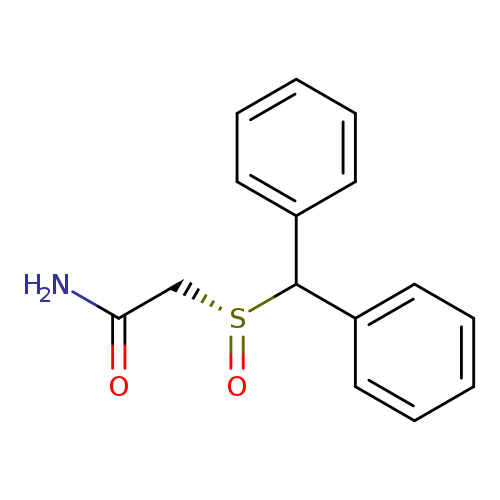
R MODAFINILCatalog No.:AA008XXH CAS No.:112111-43-0 MDL No.:MFCD09841055 MF:C15H15NO2S MW:273.3501 |
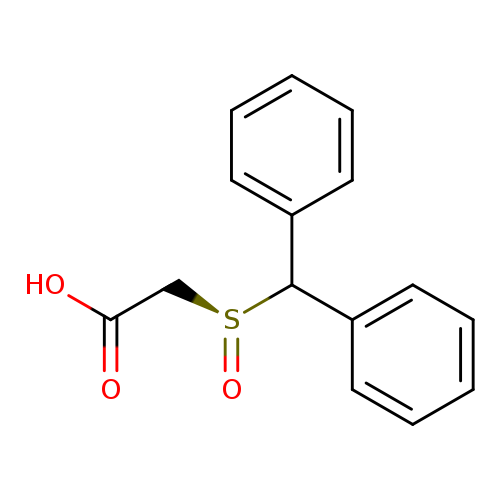
(S)-(+)-Modafinic acidCatalog No.:AA007CBL CAS No.:112111-44-1 MDL No.:MFCD15071279 MF:C15H14O3S MW:274.3349 |
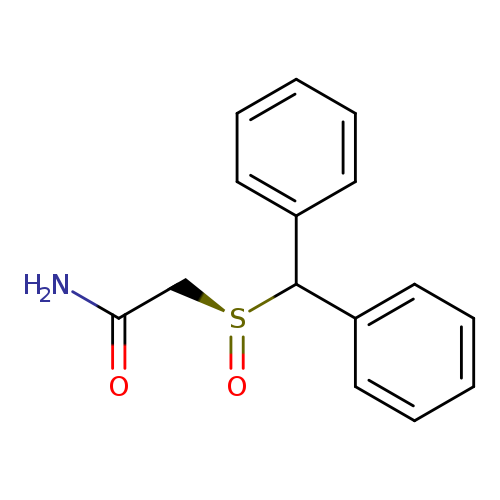
(S)-ModafinilCatalog No.:AA008XXJ CAS No.:112111-47-4 MDL No.:MFCD09841056 MF:C15H15NO2S MW:273.3501 |
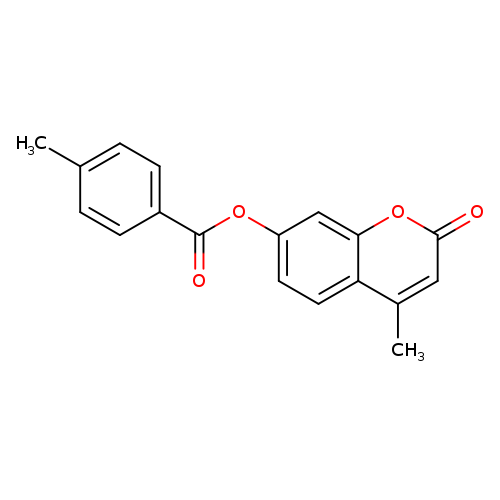
4-methyl-2-oxo-2H-chromen-7-yl 4-methylbenzoateCatalog No.:AA00IZJY CAS No.:112121-60-5 MDL No.:MFCD00169260 MF:C18H14O4 MW:294.3014 |
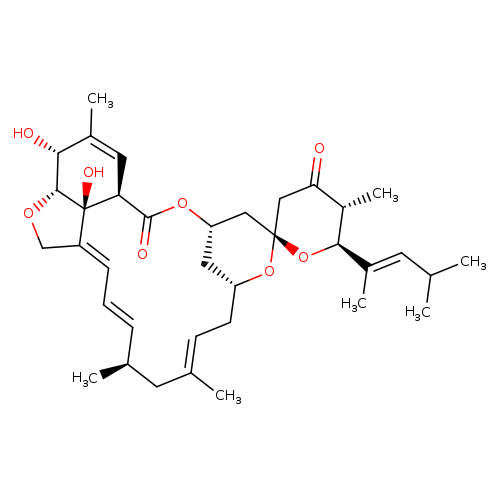
Milbemycin B,5-O-demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-oxo-, [6R,25S(E)]-Catalog No.:AA01CBYQ CAS No.:112124-81-9 MDL No.: MF:C36H50O8 MW:610.7774 |
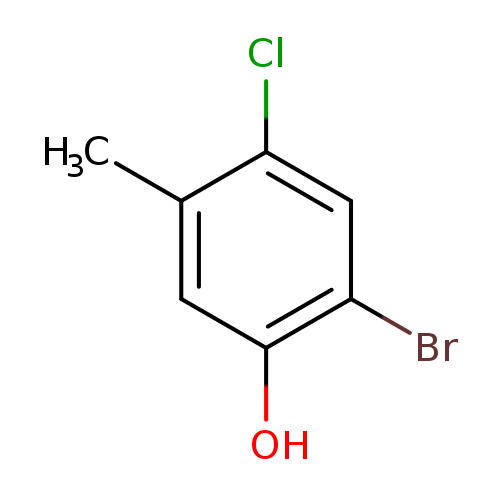
2-Bromo-4-chloro-5-methylphenolCatalog No.:AA01DLUZ CAS No.:112135-31-6 MDL No.:MFCD00029749 MF:C7H6BrClO MW:221.4789 |
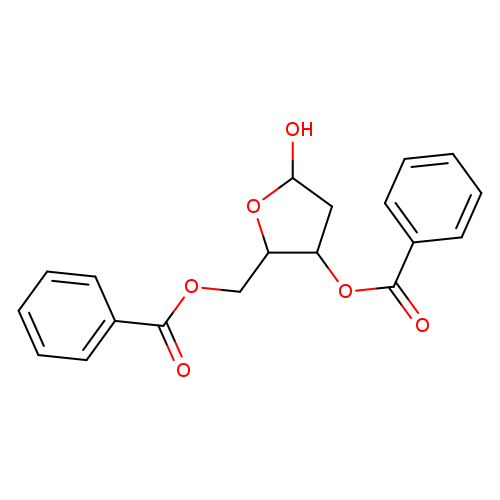
2-Deoxy-3,5-di-O-benzoylribofuranoseCatalog No.:AA008VZ9 CAS No.:112137-63-0 MDL No.:MFCD28898714 MF:C19H18O6 MW:342.3426 |
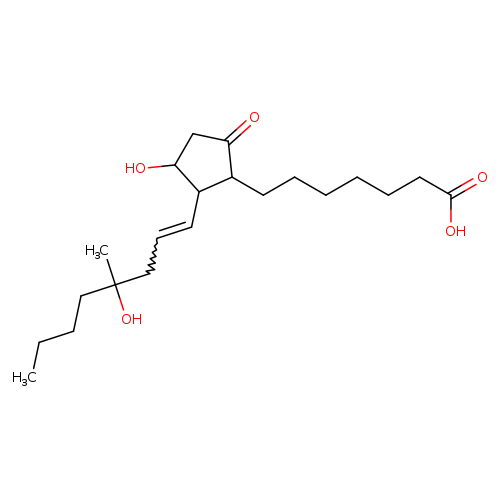
(+/-)-15-DEOXY-[16RS]-16-HYDROXY-16-METHYLPROSTAGLANDIN E1Catalog No.:AA008RHA CAS No.:112137-89-0 MDL No.:MFCD00869984 MF:C21H36O5 MW:368.5075 |
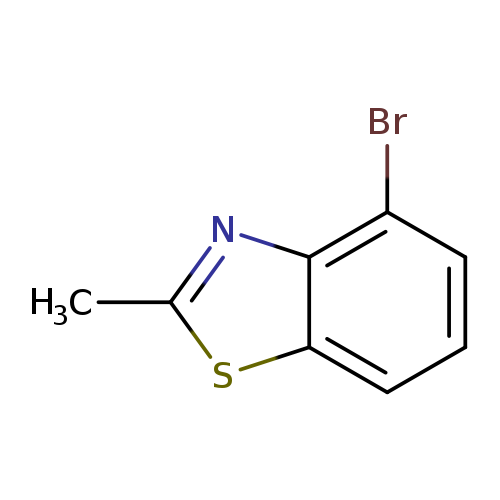
4-Bromo-2-methylbenzo[d]thiazoleCatalog No.:AA007UDB CAS No.:112146-10-8 MDL No.:MFCD11217352 MF:C8H6BrNS MW:228.1089 |
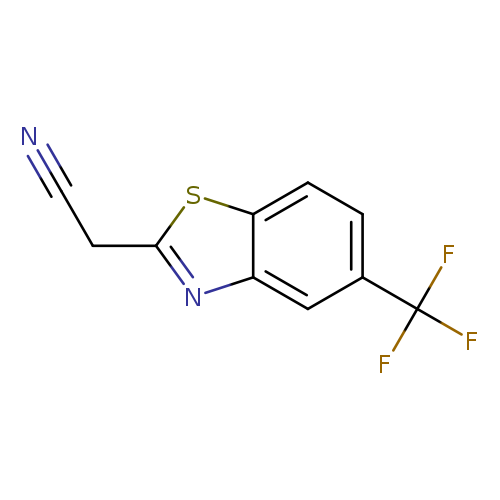
2-[5-(trifluoromethyl)-1,3-benzothiazol-2-yl]acetonitrileCatalog No.:AA019KJN CAS No.:112146-11-9 MDL No.:MFCD02073523 MF:C10H5F3N2S MW:242.2203 |
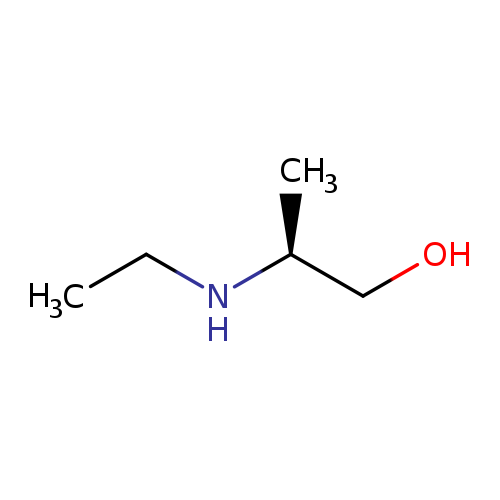
(2S)-2-(ethylamino)propan-1-olCatalog No.:AA01AM2I CAS No.:112150-26-2 MDL No.:MFCD19217977 MF:C5H13NO MW:103.1628 |
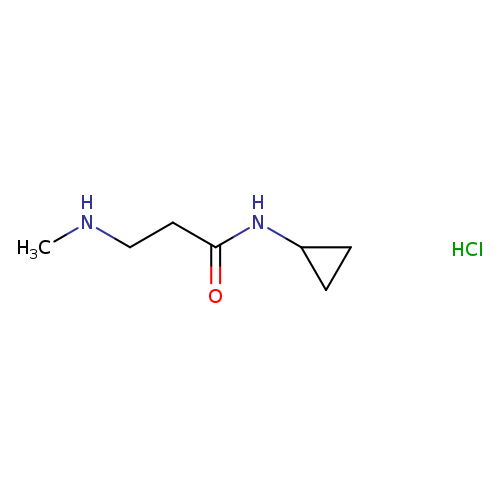
N-cyclopropyl-3-(methylamino)propanamide hydrochlorideCatalog No.:AA01AHH6 CAS No.:1121527-41-0 MDL No.:MFCD18785501 MF:C7H15ClN2O MW:178.6598 |
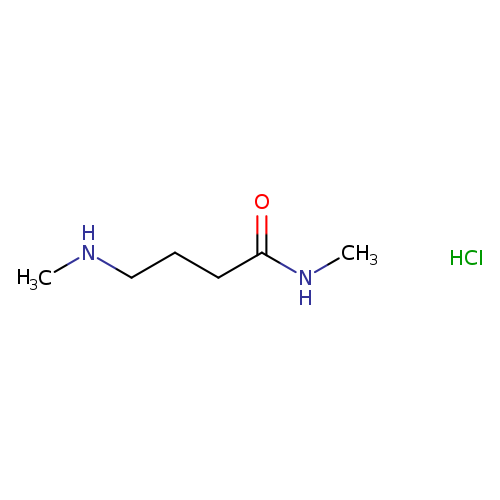
N-methyl-4-(methylamino)butanamide hydrochlorideCatalog No.:AA01A2AB CAS No.:1121527-44-3 MDL No.:MFCD22578524 MF:C6H15ClN2O MW:166.6491 |
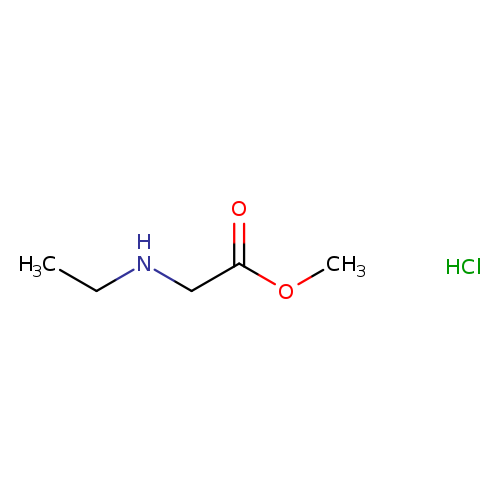
Methyl 2-(ethylamino)acetate hydrochlorideCatalog No.:AA00HC9Z CAS No.:1121527-61-4 MDL No.:MFCD16990638 MF:C5H12ClNO2 MW:153.6073 |
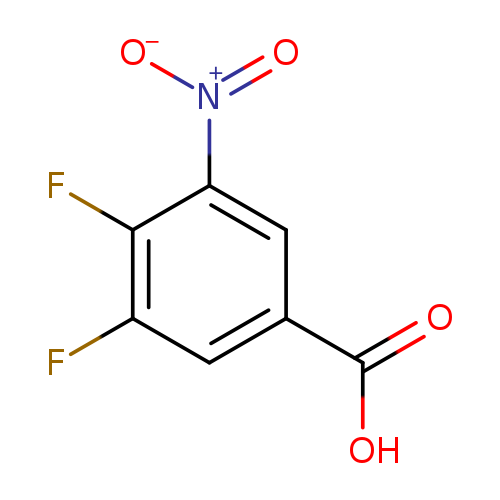
3,4-Difluoro-5-nitrobenzoic acidCatalog No.:AA008UIL CAS No.:1121583-51-4 MDL No.:MFCD15144672 MF:C7H3F2NO4 MW:203.0998 |
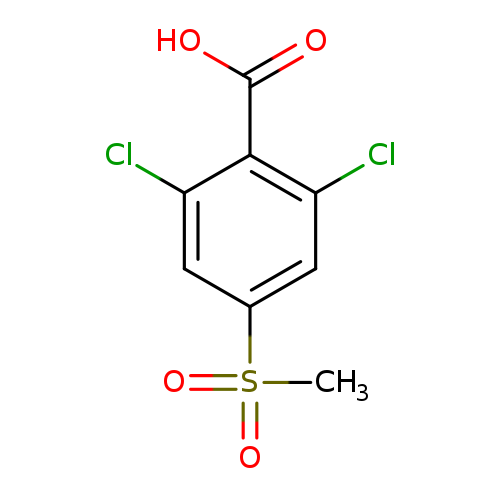
2,6-dichloro-4-methanesulfonylbenzoic acidCatalog No.:AA01A15J CAS No.:1121585-09-8 MDL No.:MFCD09999670 MF:C8H6Cl2O4S MW:269.1018 |
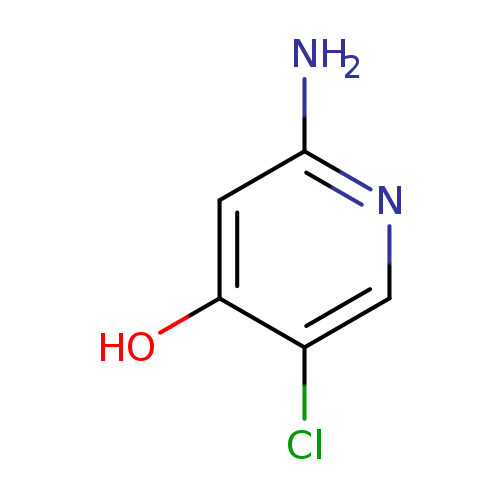
2-Amino-5-chloropyridin-4-olCatalog No.:AA019SA7 CAS No.:1121585-12-3 MDL No.:MFCD07787431 MF:C5H5ClN2O MW:144.5590 |
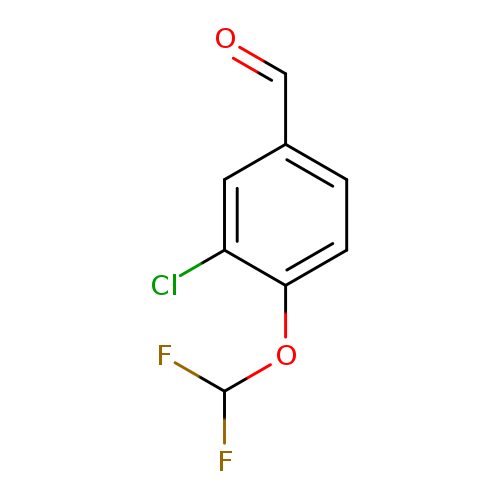
3-Chloro-4-(difluoromethoxy)benzaldehydeCatalog No.:AA01AGZ1 CAS No.:1121585-21-4 MDL No.:MFCD16075291 MF:C8H5ClF2O2 MW:206.5739 |
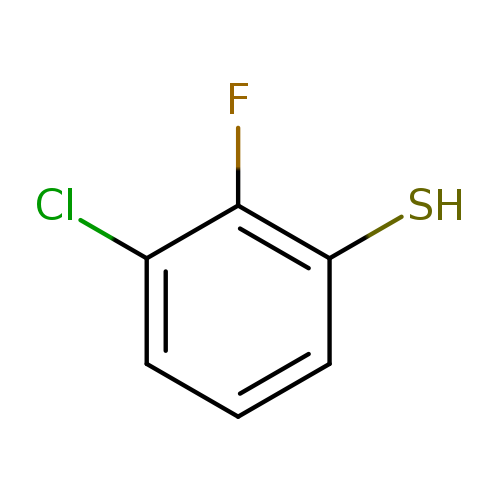
3-Chloro-2-fluorobenzenethiolCatalog No.:AA008XXB CAS No.:1121585-29-2 MDL No.:MFCD12026158 MF:C6H4ClFS MW:162.6124 |
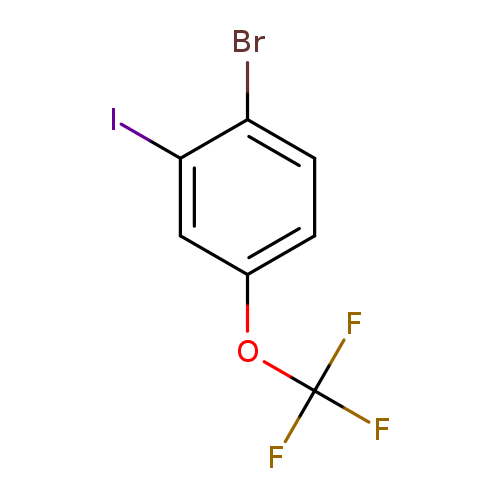
1-Bromo-2-iodo-4-(trifluoromethoxy)benzeneCatalog No.:AA007UD2 CAS No.:1121586-26-2 MDL No.:MFCD12025452 MF:C7H3BrF3IO MW:366.9018 |
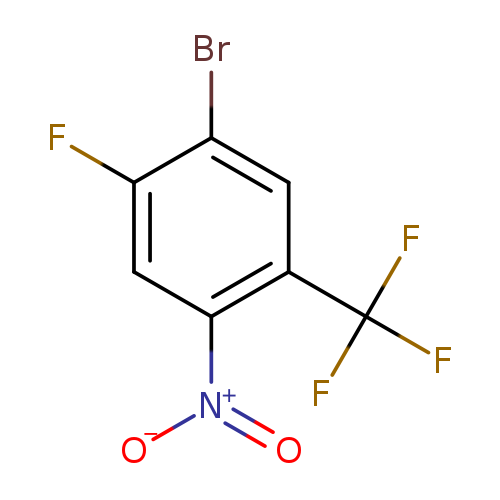
1-Bromo-2-fluoro-4-nitro-5-(trifluoromethyl)benzeneCatalog No.:AA007UD1 CAS No.:1121586-27-3 MDL No.:MFCD12032178 MF:C7H2BrF4NO2 MW:287.9939 |
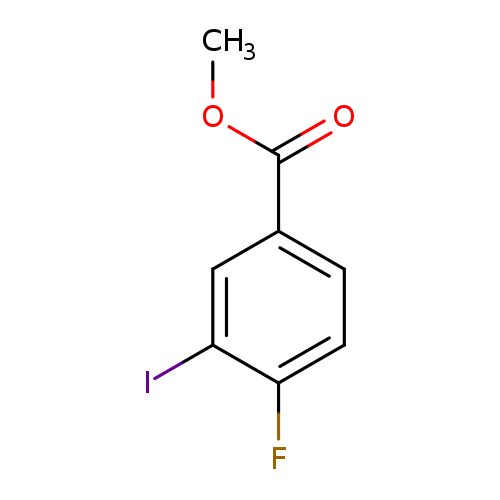
Methyl 4-fluoro-3-iodobenzoateCatalog No.:AA007C2D CAS No.:1121586-29-5 MDL No.:MFCD11870177 MF:C8H6FIO2 MW:280.0349 |
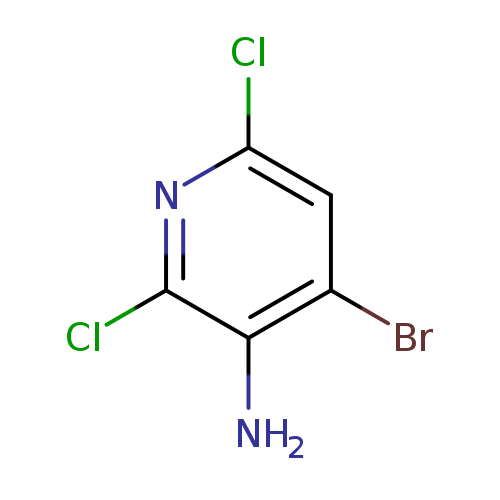
4-Bromo-2,6-dichloropyridin-3-amineCatalog No.:AA00385N CAS No.:1121586-37-5 MDL No.:MFCD12546348 MF:C5H3BrCl2N2 MW:241.9007 |
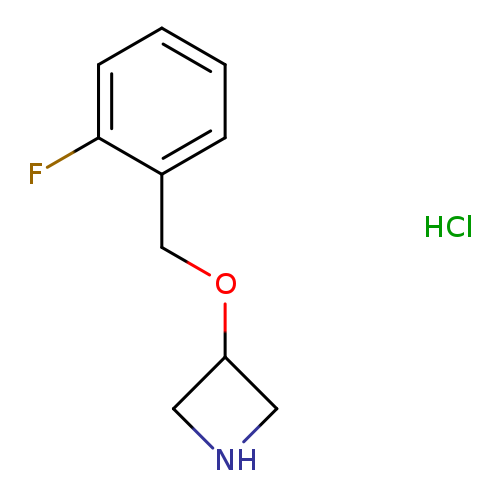
3-[(2-Fluorobenzyl)oxy]azetidine hydrochlorideCatalog No.:AA008VFL CAS No.:1121589-53-4 MDL No.:MFCD11874542 MF:C10H13ClFNO MW:217.6677 |
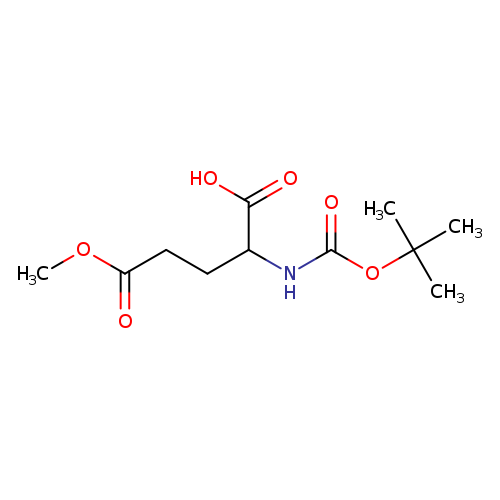
2-([(tert-Butoxy)carbonyl]amino)-5-methoxy-5-oxopentanoic acidCatalog No.:AA019EII CAS No.:112159-16-7 MDL No.:MFCD03940182 MF:C11H19NO6 MW:261.2717 |
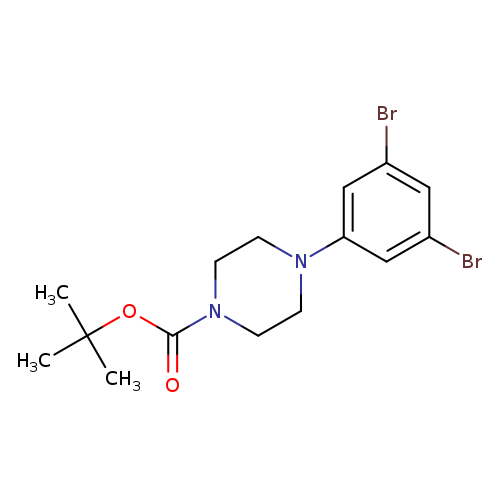
1-(4-Boc-Piperazino)-3,5-dibromobenzeneCatalog No.:AA00838J CAS No.:1121596-44-8 MDL No.:MFCD11872514 MF:C15H20Br2N2O2 MW:420.1395 |
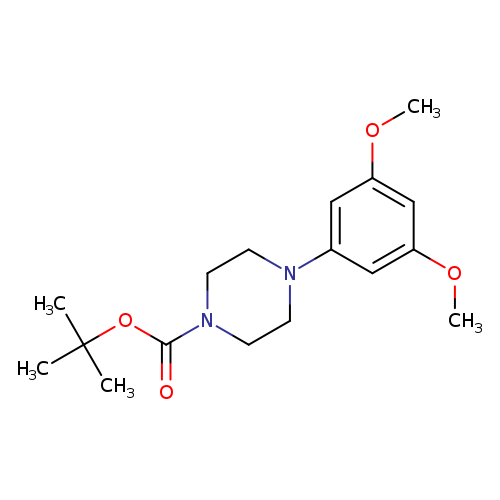
tert-butyl 4-(3,5-dimethoxyphenyl)piperazine-1-carboxylateCatalog No.:AA00HCA2 CAS No.:1121596-71-1 MDL No.:MFCD11872593 MF:C17H26N2O4 MW:322.3993 |
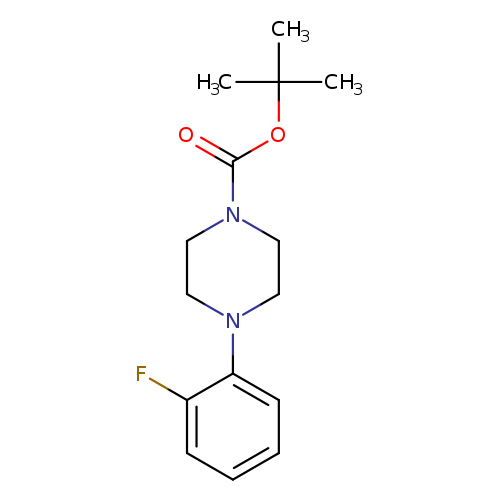
tert-Butyl 4-(2-fluorophenyl)piperazine-1-carboxylateCatalog No.:AA00HCA5 CAS No.:1121599-78-7 MDL No.:MFCD11872506 MF:C15H21FN2O2 MW:280.3378 |
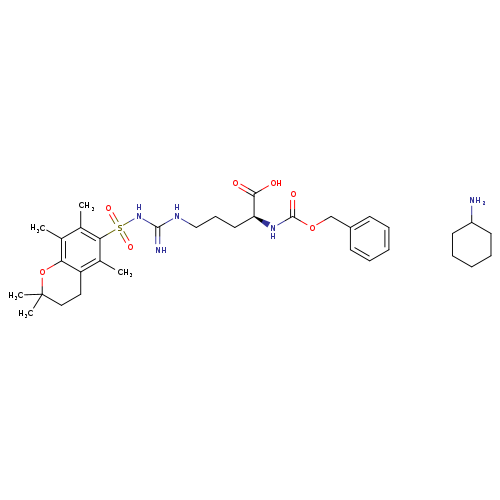
Z-Arg(PMC)-OH.CHACatalog No.:AA007UD0 CAS No.:112160-33-5 MDL No.:MFCD00153323 MF:C34H51N5O7S MW:673.8630 |
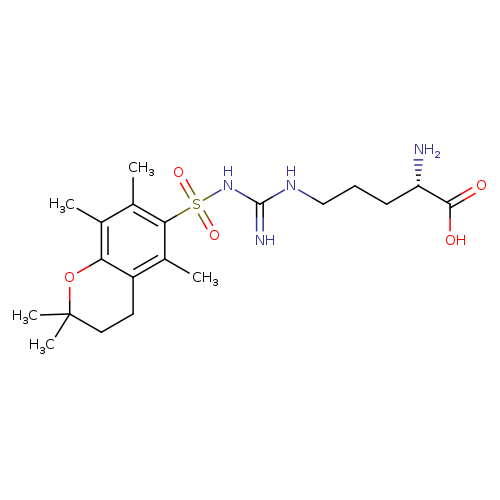
H-Arg(pmc)-ohCatalog No.:AA008Y43 CAS No.:112160-37-9 MDL No.:MFCD00153419 MF:C20H32N4O5S MW:440.5569 |
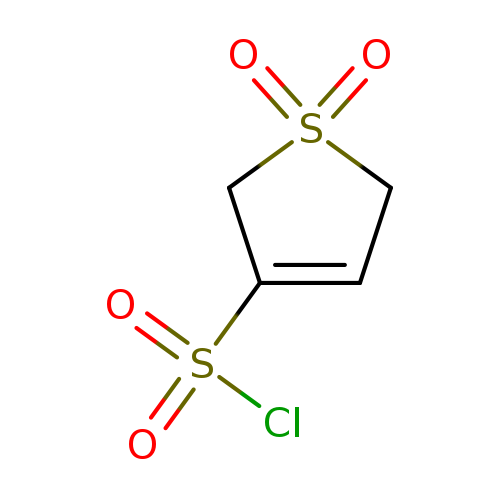
2,5-Dihydrothiophene-3-sulfonyl chloride 1,1-dioxideCatalog No.:AA008V2E CAS No.:112161-61-2 MDL No.:MFCD00196002 MF:C4H5ClO4S2 MW:216.6631 |
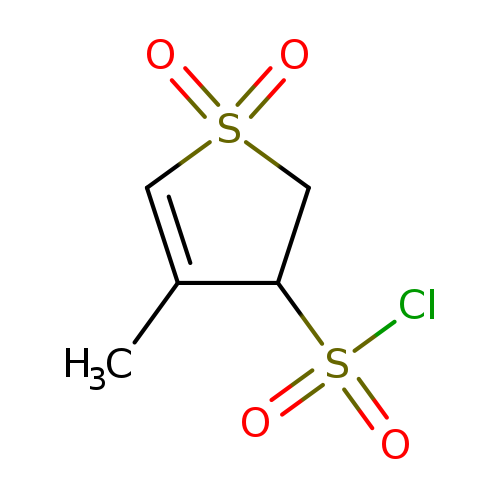
4-Methyl-2,3-dihydro-3-thiophenesulfonyl chloride 1,1-dioxideCatalog No.:AA008VI8 CAS No.:112161-65-6 MDL No.:MFCD09863871 MF:C5H7ClO4S2 MW:230.6897 |